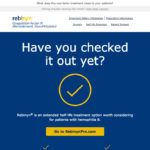
- Email 1 (Desktop)
- Email 1 (Mobile View)
- Email 2
- Email 3
- Email 4
Email 1, subject line 1: A new treatment option for your patients
Sent on: Nov 11, 2017 at 10am EST
Same email, resent:
Email 1, subject line 2: FDA Approved for 2018: Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)
Sent On: Jan 21, 2018 at 4pm EST
Email 2 subject line: Now available: Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)
Sent on: Feb 6, 2018 and Feb 11, 2018
Email 3 subject line: Now available: Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)
Sent on: Feb 26, 2018 and Mar 5, 2018
Email 4 subject line: An intro to Rebinyn® (Coagulation Factor IX [Recombinant], GlycoPEGylated)
Sent on: Apr 3, 2018 and Apr 9, 2018